Welcome Letter
Most people think of bacteria as villains that make us sick, but we see these microbes as the next heroes of global health. They play a vital role in our immune system, metabolism, and cognitive function. Over the next decade, harnessing the therapeutic potential of the microbiome will be key to solving urgent global health challenges including malnutrition, antimicrobial resistance, depression, as well as autoimmune and metabolic disorders.
In 2022, OpenBiome and microbiome biotech companies took major steps in establishing this new, promising field of medicine. This past November, the U.S. Food and Drug Administration announced the approval of the first microbiome-based therapeutic for recurrent C. difficile infections. This major milestone—made possible in part by our pioneering work with investigational fecal transplants—is a win for patients who will now have access to additional treatment options. The approval also validates the efforts of the microbiome field that is developing new approaches to address unmet medical needs.
To continue scaling the microbiome field to new heights, OpenBiome has launched a Malnutrition Program, which brings together a coalition of scientists advancing new approaches to care. By developing novel microbiome-based therapies to augment the standard treatment of nutrient-enhanced foods, we aim to prevent 2 million child deaths each year.
I hope you will join us in recognizing microbiome science as a solution to urgent and previously intractable public health threats. Over the past decade, our mission has been made possible by patients and their families, physicians, and scientists. We are grateful to have worked with such caring, bold, and innovative collaborators, and look forward to years of continued progress. Together, we can accelerate research and drive innovation to improve health worldwide.



Pioneering a New Field of Medicine: OpenBiome is creating a new relationship between microbes and healthcare by exploring how bacteria keep us healthy as well as how they can be used to prevent and treat disease.
Humans and bacteria have a deep and complex relationship that modern medicine is just beginning to tap into. Over thousands of years, we have coevolved with the microbes inside us and come to depend on them for our health. Bacteria help us digest our food, train our immune system, and even modulate brain function.
Because of its importance to our health, the microbiome is often referred to as a “hidden organ.”
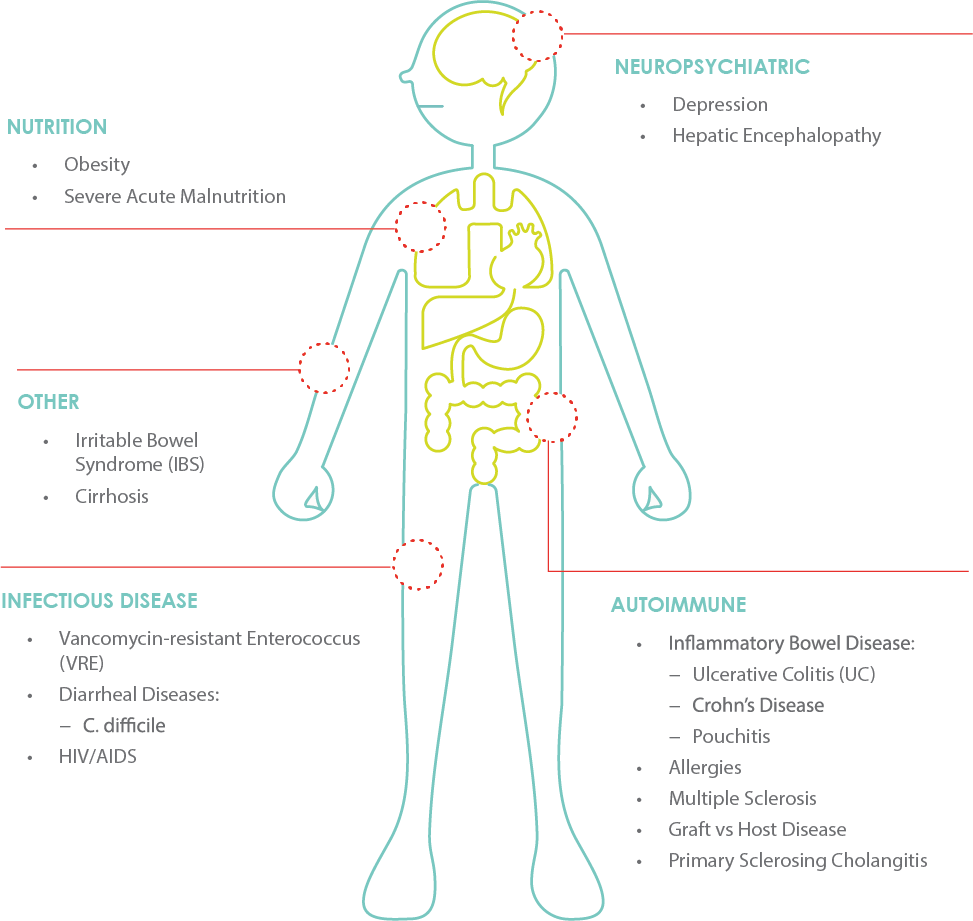
The success that OpenBiome and our clinical partners have had in using investigational fecal transplants to treat antibiotic-resistant C. difficile infections has inspired scientists to test whether engineering the microbiome can treat a wide range of diseases including malnutrition, food allergies, and neuropsychiatric disorders.
A History of Exploration:
Since 2013, OpenBiome has supported more than 40 clinical trials and single patient case studies, and treated more than 65,000 patients with recurrent C. difficile infections. Our clinical trials portfolio spanned autoimmune, infectious, metabolic, and neuropsychiatric disease. We are currently focusing our expertise on developing a micorobiome-based therapy for malnutrition.
"When we begin to understand our microbiomes, our symbionts, our inner ecosystems, our staggering multitudes–every walk bristles with opportunities for discovery."
— Ed Yong, Author of I Contain Multitudes
OpenBiome was originally founded as a stool bank in 2013. For the past decade, we have been turning healthy people’s poo, and the bacteria it contains, into investigational medical treatments known as fecal microbiota transplantation (FMT).
To date, we have provided over 65,000 FMT preparations for patients with antibiotic-resistant C. difficile infections (CDI).
This work proved that microbes could treat incurable illness and laid the foundation for a new field of medicine and biotech innovation.
Watch a testimonial from a patient who received an investigational FMT from OpenBiome.

2022 Impact: By the Numbers
C. difficile infects about 400,000 people in the United States each year leading to approximately 20,000 deaths.
![Sick_Main_Comp[1] Sick_Main_Comp[1]](https://openbiome.org/wp-content/uploads/Sick_Main_Comp1.gif)
65,000+ Patients have received urgently needed investigational FMT preparations distributed by OpenBiome.
![CHARACTERS_MAIN_COMP[1] CHARACTERS_MAIN_COMP[1]](https://openbiome.org/wp-content/uploads/CHARACTERS_MAIN_COMP1.gif)
2022 Highlights

The Global Impact of Malnutrition
Severe acute malnutrition (SAM) results in nearly 2 million preventable child deaths every year. OpenBiome's Malnutrition Program is bringing together a coalition of scientists to advance new therapies for this urgent public health threat.
![BOY_Thin_Main_Comp[1] BOY_Thin_Main_Comp[1]](https://openbiome.org/wp-content/uploads/BOY_Thin_Main_Comp1.gif)
children under 5 years of age are affected by wasting (defined as low weight-for-height).
![BOY_Short_Main_Comp[1] BOY_Short_Main_Comp[1]](https://openbiome.org/wp-content/uploads/BOY_Short_Main_Comp1-1.gif)
children under 5 years of age are affected by stunting (defined as low height-for-age).
![PLATE_Main_Comp[1] PLATE_Main_Comp[1]](https://openbiome.org/wp-content/uploads/PLATE_Main_Comp1.gif)
of childhood deaths under 5 years of age are linked to undernutrition.
Going Beyond Standard Treatments
Children with severe acute malnutrition (SAM) typically receive antibiotics and nutrient-enhanced foods. However, over a third of patients don’t respond to standard treatment. These children are likely lacking gut microbes required to absorb and maximize the nutrition that is available to them.
OpenBiome and our research collaborators are identifying the crucial microbes that children with SAM are missing and developing live biotherapeutic therapies to be administered alongside nutrient-enhanced foods.
"As a nonprofit leading the way in translating microbiome research into patient care, we believe that enhancing childhood malnutrition outcomes could be one of our field's most significant impacts. With the potential to save thousands of lives annually, we feel an urgent responsibility to apply this science and rigorously evaluate the effectiveness of microbiome-directed interventions."
- Dr. Majdi Osman, Chief Medical Officer at OpenBiome

Spotlight on THRIVE
- In 2019, OpenBiome embarked on an inaugural global health study, THRIVE (Transfer of Healthy Gut Flora for Restoration of Intestinal Microbiota via Enema), with the University of Cape Town, South Africa.
- THRIVE is the first clinical trial of FMT in Africa, and the first to evaluate microbiome manipulation in children with malnutrition. In our pilot study, we observed that the intervention was safe and well tolerated, improved the gut microbiome diversity of children with SAM, and improved biomarkers of gut inflammation and nutritional recovery in children.
- The study has the potential to catalyze novel therapeutic avenues to address malnutrition and improve outcomes for millions of children every year.

About Us
OpenBiome is a pioneering nonprofit that accelerates microbiome science to improve health for all. We partner with leading researchers, clinicians, and innovators to ensure patient access to novel and affordable microbiome therapeutics.
Since its founding at MIT in 2013, OpenBiome has provided investigational fecal microbiota transplantation (FMT) treatments to more than 65,000 patients with recurrent C. difficile infections and supported over 40 studies investigating how the microbiome affects human health.

![Man_Bottle_Main_Comp[1] Man_Bottle_Main_Comp[1]](https://openbiome.org/wp-content/uploads/Man_Bottle_Main_Comp1.gif)
![2023_graphics_-08[1] 2023_graphics_-08[1]](https://openbiome.org/wp-content/uploads/2023_graphics_-081.jpg)
![2023_graphics_-25[1] 2023_graphics_-25[1]](https://openbiome.org/wp-content/uploads/2023_graphics_-251.jpg)